![[Translate to English:]](/fileadmin/_processed_/a/d/csm_NTMedicalSolutions_2d22fcb66f.jpeg)
Development of safe medical devices
NewTec offers comprehensive support for the development of medical devices
There are special requirements on medical devices in terms of safety and usability. With mobile healthcare, telemedicine and the networking in the IoMT (Internet of Medical Things), the relevance of information security is increasing. The regulatory requirements for engineering are correspondingly high and are constantly growing, e.g. in the form of the new EU Medical Device Regulation (MDR for short).
What’s more, ever shorter innovation cycles force manufacturers to reduce their time to market. NewTec helps you to overcome these challenges. With technology consulting, engineering services, software solutions and hardware solutions, we have been a competent partner for well-known producers of medical devices for many years.

Electronics and software development of medical technology
NewTec provides comprehensive support in the development of medical devices with worldwide approval up to class 2b and in the development of medical apps. Experienced engineers are at your side for the entire development and product lifecycle – from technology consulting, system and requirements engineering, software and hardware development through to the design of intuitive and safe interfaces.
The relevant FDA specifications and the ISO and IEC standards for the development of medical systems must be considered at all process stages. Thanks to its certification in accordance with DIN EN ISO 13485 as an extension to ISO 9001, NewTec is a safe and reliable development partner.
Safety for medical technology
As specialists in functional safety, we use our expertise, sustainable safety concepts and special hardware and software to ensure that your medical devices meet all the requirements of safety-related standards such as DIN EN ISO 14971, DIN EN 60601 or IEC 62304. From technology consultancy and requirements for implementation through to verification and validation, we ensure certified and proven processes for the safety of your products. To achieve the greatest possible levels of safety and reproducibility, each individual step in the project phase is documented in full.
Security in medical technology
Mobile Health and the Internet of Medical Things place high demands on the information security of networked medical devices (data protection and protection against attacks). There are corresponding regulatory requirements for security, not least because of the MDR and FDA. However, security engineering is uncharted territory for many manufacturers of medical devices.
NewTec supports manufacturers in the development of new medical devices with security analyses and security engineering, including all normative requirements like IEC 60601-4-5, IEC 62443 or ISO/IEC 27001. We’ll advise you on protection against sabotage attacks and external manipulation and check whether and how legacy products can be made fit for the security requirements of networked devices. And we develop safe, data protection-compliant applications.
Usability of medical devices
Errors in the operation of medical devices can endanger patients and the users. Simple, safe and intuitive operation is absolutely essential to avoid errors – especially in stress situations.
NewTec provides comprehensive usability engineering, taking into account relevant standards such as DIN EN 62366 and DIN EN 60601-1-6. We determine the needs of the users depending on the situation in detailed application analyses. Based on these analyses, we develop intuitive user interfaces that minimise susceptibility to operating errors as much as possible. To ensure safe operation, even in a wide variety of countries with their own specific requirements, NewTec carries out usability studies taking linguistic and cultural features into account.

Platforms and solutions for medical applications
With our customised solutions and platforms for different areas, we succeed in implementing your medical technology projects quickly and safely.

Wireless solutions
With NewTec’s safe and reliable wireless solutions (NTReliableWirelessSolutions), components can be flexibly and safely connected using different wireless technologies. Our innovative wireless network topology solution NTStarEcho enables the networking of IoMT devices critical to security with low latency and in such a way that data transfer is highly secure. As an associate member of Bluetooth SIG, our experts provide you support with targeted workshops and in product development.

Platform for secure IoMT applications
With NTSecureCloudSolutions, NewTec is providing a comprehensive solution and service package for innovative and secure IoMT applications. Applications from secure mobile health apps through to patient data management systems can be implemented on the basis of certified hardware solutions and software solutions.

Safety platforms
Development platforms such as NTMicroDrive for safely controlling electric motors and the safety engineering platforms SafeFlex (FPGA-based) and NTSafeFlex STM32 (MicroController-based) reduce the cost of development of safety-related applications and shorten the time to market.
Practical MDR Support
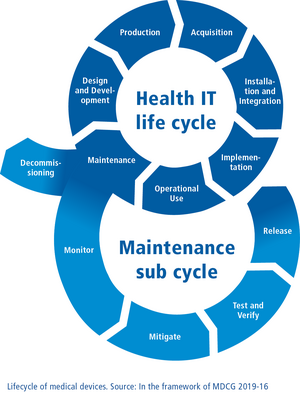
The European Medical Devices Regulation EU 2017/745 (MDR) is intended to create more safety for patients. In practice, however, specifications on risk and quality management, clinical assessments and follow-ups (post-market surveillance) and on the continuous updating of technical documentation represent enormous challenges for manufacturers.
Based on our decades of development experience, our expertise in the implementation of relevant regulations and a comprehensive knowledge of MDR and FDA regulations, we assist manufacturers in the MDR-compliant development of medical devices with individual training, consultation and engineering. When it comes to legacy products, we help make them fit for MDR approval and in creating and updating MDR-compliant technical documentation.

